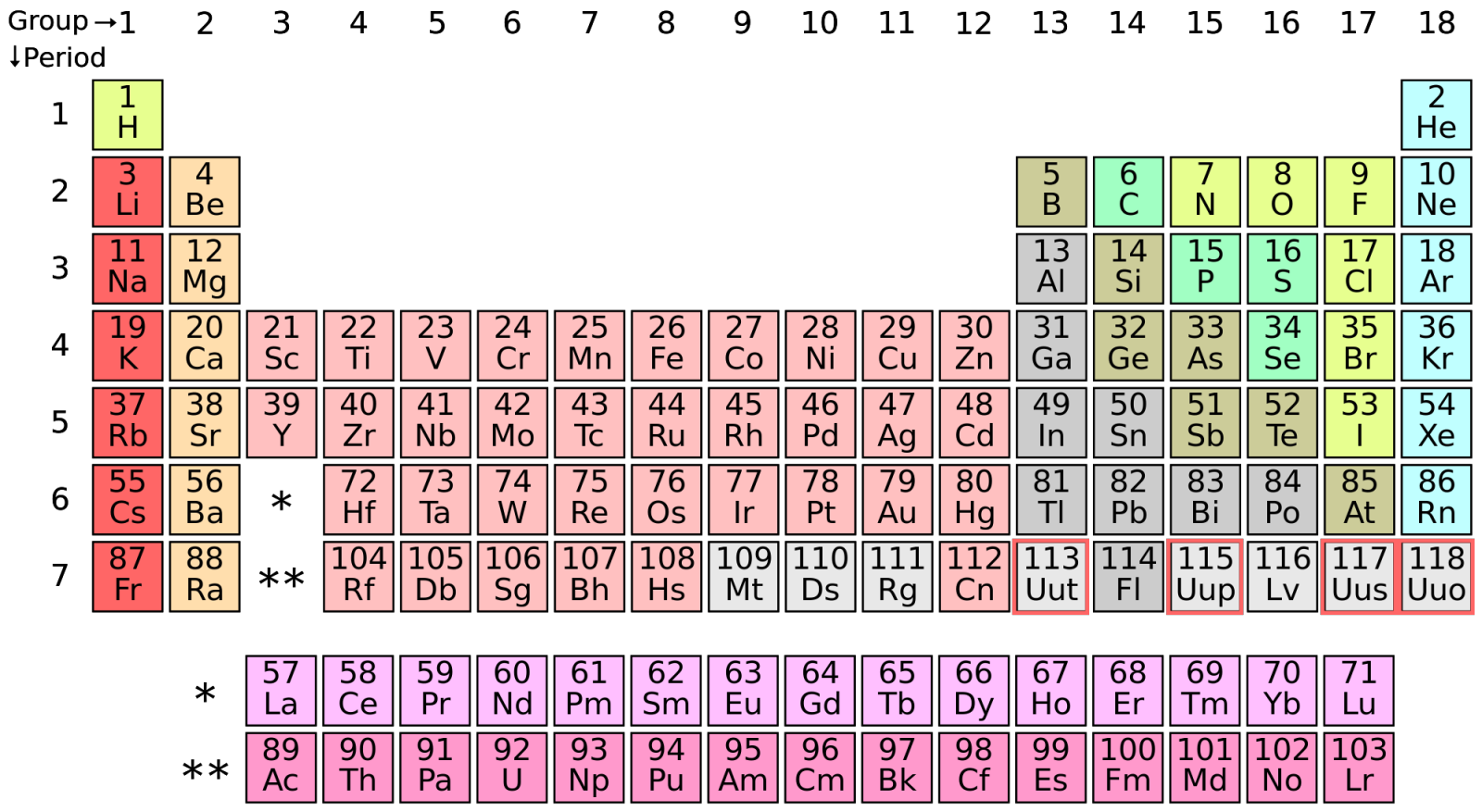
The periodic table gets 4 new elements


Get involved with our crowdsourced digital platform to deliver impact at scale
Stay up to date:
Chemical and Advanced Materials
This article is published in collaboration with Quartz.
If science has an iconic image, it is that of the periodic table of elements.
To keep up with the Agenda subscribe to our weekly newsletter.
Author: Akshat Rathi is a reporter for Quartz in London.
Image: A sign showing titanium on the periodic table of elements is seen at the Nobel Biocare manufacturing facility in Yorba Linda, California. REUTERS/Mike Blake.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Related topics:
The Agenda Weekly
A weekly update of the most important issues driving the global agenda
You can unsubscribe at any time using the link in our emails. For more details, review our privacy policy.
More on Industries in DepthSee all
Abhay Pareek and Drishti Kumar
April 23, 2024
Charlotte Edmond
April 11, 2024
Victoria Masterson
April 5, 2024
Douglas Broom
April 3, 2024
Naoko Tochibayashi and Naoko Kutty
March 28, 2024