mRNA vaccines - here's everything you need to know

What's inside an mRNA vaccine vial? And how does it differ from other vaccines? Image: Daniel Schludi, Unsplash
Kevin Doxzen
Hoffmann Fellow, Precision Medicine and Emerging Biotechnologies, World Economic Forum LLC
Explore and monitor how COVID-19 is affecting economies, industries and global issues

Get involved with our crowdsourced digital platform to deliver impact at scale
Stay up to date:
COVID-19
Listen to the article
- In December 2020, the US FDA issued emergency use authorization for mRNA vaccines developed by Pfizer-BioNTech and Moderna, making them the first mRNA vaccines available to the public.
- mRNA vaccines differ from other approaches – teaching our cells to become autonomous vaccine production plants – and offering benefits of speed of production and the ability to keep up with new variants.
- mRNA vaccines hold promise for combatting cancer and infectious diseases, such as malaria and even flu.
On 16 March 2020, the first ‘mRNA’ vaccine designed to fend off the SARS-CoV-2 virus was injected into Jennifer Haller in Seattle. This date marked the beginning of human clinical trials to test the safety of a new COVID-19 vaccine from the biotechnology company Moderna.
The shot was administered only 66 days after the sequence of the virus’s genome was released to the world, an unprecedented turnaround time that many people hope will foreshadow the future of vaccine development and international collaboration. In December 2020, the US Food and Drug Administration issued emergency use authorization for mRNA vaccines developed by Pfizer-BioNTech and Moderna, making them the first mRNA vaccines available to the public.
So what was inside that vaccine vial? How do mRNA vaccines differ from other approaches, and what’s their promise for the future?
Cells, RNA, DNA, genetic blueprints and proteins
In the cell, the main job of RNA is to convert the information stored in DNA – our genetic blueprint – into proteins. This task is carried out by a specific type of RNA called “messenger” RNA, or mRNA.
Deciphering the cellular role of mRNA was the result of decades of research during the mid-20th century, performed by multiple international research teams. In the 1990’s, scientists figured out how to introduce custom mRNA into cells, directing the cells to make specific proteins. This discovery would eventually pave the way for developing mRNA vaccines.
mRNA: teaching our cells to make their own vaccine
Vaccines work by training our bodies to recognize invading viruses. Traditional vaccines perform this task by introducing a dead, inactive, or modified portion of a virus into our body so that our immune system can learn to recognize and fight this foreign invader.
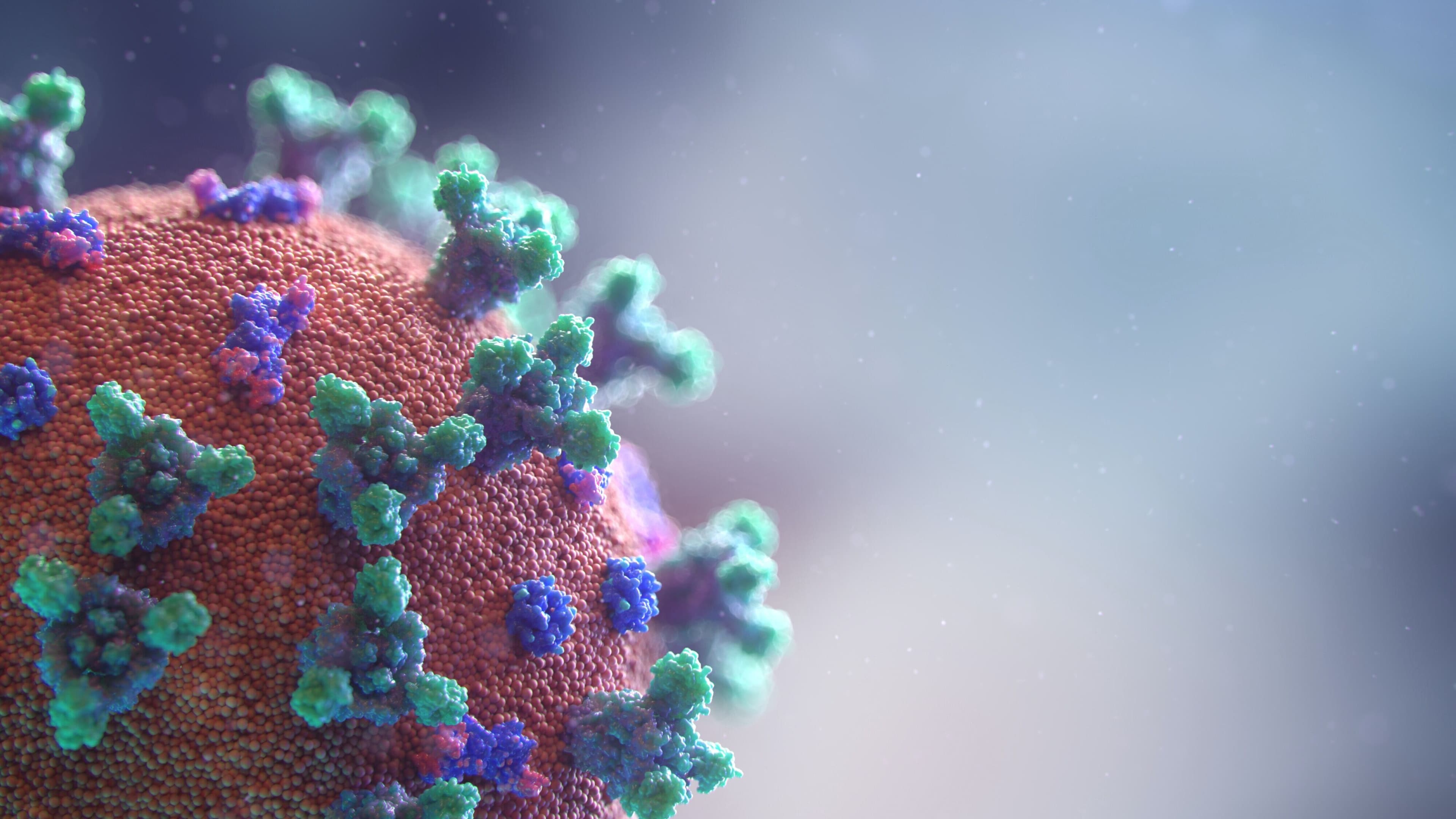
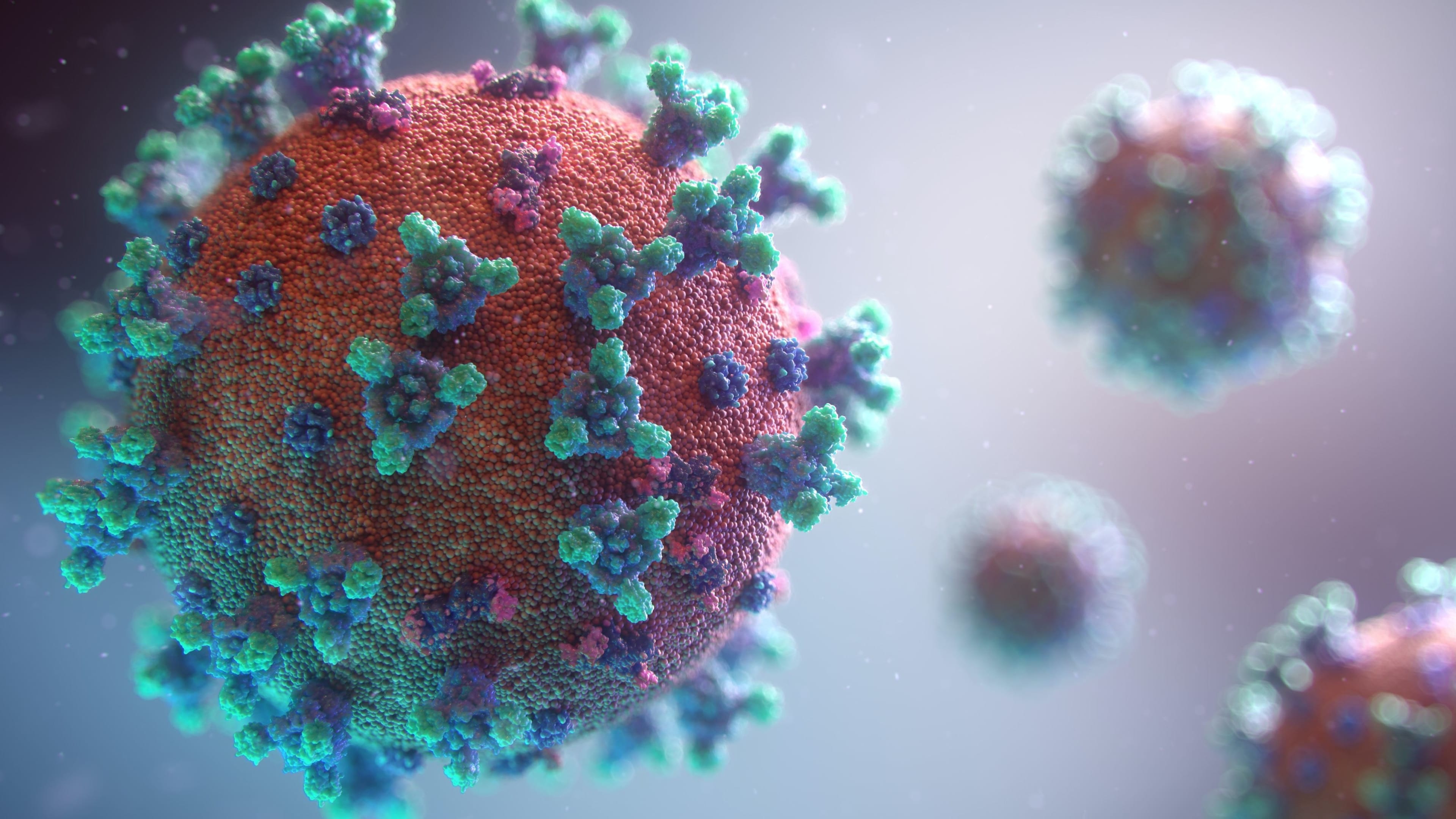
In the case of Moderna’s and Pfizer-BioNTech's mRNA vaccines, we are not injected with a whole virus or even a piece. Instead, we are supplied mRNA that instructs our cells to make a version of the SARS-CoV-2 spike protein. These instructions teach our cells to become their own vaccine manufacturing plants.
In the lab, scientists create synthetic mRNA containing the spike protein sequence. This encoded information is delivered through the jab, and it instructs some of our cells to manufacture spike proteins. The spike proteins trigger our immune cells to assemble antibodies capable of recognizing them. If the SARS-CoV-2 virus, the virus behind COVID-19, infects a vaccinated person, then the trained antibodies sound an alarm, leading to an immune response to fend off the infection.
The fundamental idea behind using a vaccine to teach a body’s immune system dates back over 200 years, but the use of mRNA is a recent development. Compared to other methods, mRNA leads the way in both speed and flexibility.
The pros of mRNA vaccines
In theory, the underlying technology behind mRNA vaccines is adaptable, allowing for quick updates as new viral mutations (variants) evolve or whole new viruses are discovered. Since mRNA vaccines are based on sequences of viral proteins, making a new vaccine could simply involve changing the mRNA sequence if you know what protein you want to make.
mRNA vaccines are also quicker and more reliably manufactured than traditional vaccines. For Moderna, the entire process—from vaccine design to manufacturing to shipment—took only 7 weeks. Although design and production of mRNA vaccines may take only weeks, necessary clinical trials to evaluate safety and efficacy still require several months of testing.
In contrast, other forms of vaccines use disabled or weakened viruses (i.e. measles and hepatitis A). These can take months or years to design. Manufacturing enough viruses to vaccinate a large portion of society can be a cumbersome process. For example, the influenza virus is grown inside fertilized chicken eggs, which are obtained from sterile laying facilities.
The cons of mRNA vaccines
Despite the benefits of mRNA vaccines, there are still risks and unknowns. They are not as stable at high temperatures, making packaging and distribution difficult. Long-term storage and delivery of vaccines is vital as governments seek to vaccinate rural and remote communities. This requires global infrastructure investments, workforce training and last mile coordination.
Second, although clinical trials and early studies of these vaccines in real-world use have shown largely positive results, the long-term effects are still unknown.
Promise for the future
Beyond COVID-19 and its variants, researchers are developing mRNA vaccines for other infectious diseases. Early animal testing has shown that mRNA vaccines can fend off viruses like influenza, Zika and rabies. Researchers from the University of Illinois, Chicago are engineering an mRNA vaccine for the dengue virus while research into a vaccine for malaria is gaining ground.
BioNTech recently announced the first vaccine for malaria based on mRNA technology and aims to start clinical testing by the end on 2022, while human trials for an HIV vaccine developed by Moderna are due to start soon.
In total, of the 44 ongoing clinical trials of mRNA vaccines, 23 are targeting infectious diseases.
What is the World Economic Forum doing about access to vaccines?
Prior to developing mRNA vaccines for infectious diseases, researchers and pharmaceutical companies contemplated mRNA’s potential to treat cancer. Over 20 mRNA vaccines in clinical trials are in oncology, testing mRNA as a personalized treatment tool. Ideally, doctors would identify the unique mutations present in a patient’s cancer cells and introduce those letters into an mRNA vaccine, teaching a patient’s immune system to more effectively attack cancer cells.
Promise exists for using mRNA to treat several other diseases as well. What is certain is that mRNA is poised to impact public health and precision medicine. The extent of this impact relies heavily on accessibility. The COVID-19 pandemic has prompted multistakeholder collaboration on strengthening global infrastructure, fostering public-private partnerships, and ensuring last mile delivery. The global community’s work to address current gaps and barriers in access to mRNA vaccines will provide a foundation for continued initiatives needed to ensure access to future mRNA treatments.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Related topics:
The Agenda Weekly
A weekly update of the most important issues driving the global agenda
You can unsubscribe at any time using the link in our emails. For more details, review our privacy policy.
More on COVID-19See all
Charlotte Edmond
January 8, 2024
Charlotte Edmond
October 11, 2023
Douglas Broom
August 8, 2023
Simon Nicholas Williams
May 9, 2023
Philip Clarke, Jack Pollard and Mara Violato
April 17, 2023