How to distribute a COVID-19 vaccine and build public trust

Everyday life has been changed by the pandemic. Image: REUTERS/Gleb Garanich
- The World Economic Forum’s Sustainable Development Impact Summit featured two sessions updating on progress of vaccine development and distribution.
- Leaders from pharmaceutical companies and bodies involved in the COVAX Facility – to ensure equitable distribution – discussed issues from vaccine nationalism to public trust.
The coming months are going to be crucial for vaccine development and decisions around how we distribute COVID-19 vaccines equitably, who gets them first – and how to gain the public’s trust.
These were some of the challenges discussed in two sessions at the World Economic Forum's Sustainable Development Impact Summit.
Have you read?
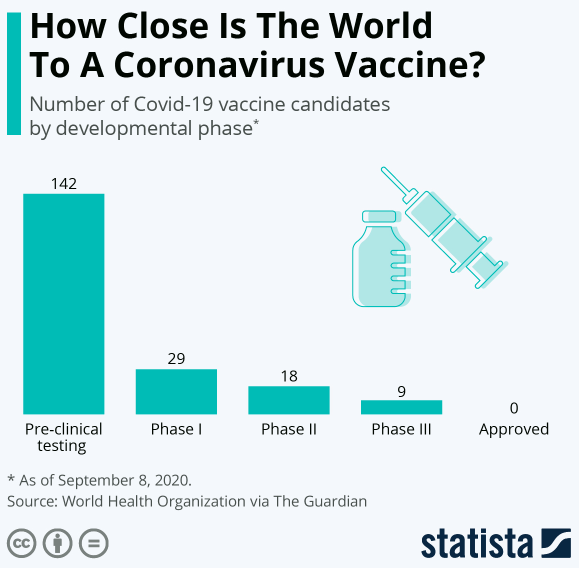
With 42 vaccine candidates in clinical trials on humans, scientists are working hard to fast-track the development of a safe and effective vaccine that would typically take decades’ work.
“We are at the beginning of the beginning,” said Sai Prasad, President of the Developing Countries Vaccine Manufacturers Network.
“We are just at the end of the first year, and we have a few candidates that are moving forward. None of them are successful yet. Time will tell – the next three to four months – as to which one of these candidates will be successful.
“That’s the end of the beginning, because once you know what’s successful, you have to manufacture that at scale. The devil is in the detail. After you manufacture, you have to get these products approved and then distribute them.”
In the first six months of the pandemic, more than 700 products for treatment or prevention of COVID-19 went into the pipeline, which is unprecedented, said Julie Gerberding, Executive Vice-President of pharmaceuticals company Merck.
She compared the response speed with that of the AIDS epidemic: “It was several years before there was even a test and it was 15 years before a highly active antiviral remedy. We’re living in an era where the science has brought possibilities.”
Antivirals, which Merck is also working on, will help. “If we can take mortality down, it buys us time... I don’t think we should put all our eggs in the vaccine basket, but clearly it’s a huge component of securing global protection and we have to prioritize it.”
Working together
Once a vaccine is successful, how can world leaders ensure against “vaccine nationalism” – and make sure it’s distributed fairly across the globe?
Gavi, The Vaccine Alliance, along with the World Health Organization and the Coalition for Epidemic Preparedness Innovations (CEPI), have created the COVAX Facility, a global initiative that brings together governments and manufacturers to ensure eventual COVID-19 vaccines reach those in greatest need.
So far, a total of 156 economies, representing nearly two-thirds of the global population, are now committed to or eligible to receive vaccines through the Facility.
What is the World Economic Forum doing about the coronavirus outbreak?
"A vaccine is the way we're going to get out of this pandemic… that is the best way we have to go back towards normal,” said Seth Berkley, Chief Executive Officer of Gavi.
“Our belief in a fast-moving pandemic is you’re not safe unless everyone is safe. What the COVAX Facility is trying to do is to get a vaccine out to all countries, rich and poor, at the same time.”
Initially, he said, this would be frontline health workers and those most at risk and then the broader population.
Gavi is working with the pharmaceutical industry to scale up production – and it is looking to have 2 billion doses available by the end of 2021.
Before COVAX, countries didn’t have the option of working together, said Richard Hatchett, Chief Executive Officer of CEPI.
“They were behaving in their own rational self-interest, which… would have been inequitable and resulted in a perpetuation of the pandemic.
“We have had to devise institutional arrangements in real time to develop and create a space for international collaboration in development, procurement and delivery. Designing a system to solve all those at one time is a huge challenge.”
He said it’s encouraging to see “emerging momentum around global solidarity and collaboration and willingness to work with us”.
Global coverage, local plans
Companies need to look at the issues of the developing world during product development, said Prasad of the Developing Countries Vaccine Manufacturers Network, particularly around vaccine storage temperature, transportation and biomedical waste disposal.
“Many companies are doing that. But if they only have a US focus, they might not see the needs of the middle-income and low-income countries.”
Gavi's Berkley said each country would need a bespoke plan for who to vaccinate first.
“In the US, it might be [people in] prisons or meat-packing plants, the elderly, but in developing countries, you might not have a high elderly population, but you might have urban slums and displaced people, so you really have to understand the local situation even though we can have global guidelines.”
Single shot
Johnson & Johnson's candidate is the latest to go into Phase 3 clinical trials and will now be studied as a single-dose vaccine on 60,000 people.
The pharmaceutical company says it’s on track to meet its goal of providing 1 billion doses of an affordable vaccine each year. It anticipates the first batches to be available in early 2021, “if proven to be safe and effective”.
Paul Stoffels, Johnson & Johnson's Chief Scientific Officer, said the first vaccines would be ready early next year – and explained the trial size was determined by transmission rate.
"To reach a statistically significant end point, we need to have significant numbers, so there's a lot of data science to see where we have to target people most at risk."
He said the company has learned from developing vaccines for the Ebola virus that single-shot vaccines are efficacious, but they will be testing booster shots at a later date.
Transparency and trust
All eyes are on the pharmaceutical companies during the development process, and once a vaccine is ready to be distributed, will the global public want to take it?
In a recent World Economic Forum-Ipsos survey of nearly 20,000 adults from 27 countries, 74% said they would get a vaccine for COVID-19.
This majority might still fall short of the number required to beat COVID-19, with just 37% strongly agreeing they would be willing.
Pascal Soriot, the CEO of AstraZeneca, said companies working on COVID-19 vaccines are looking at how to provide greater transparency without impacting on vaccine trials.
AstraZeneca, which is developing a vaccine with the University of Oxford, paused its Phase 3 trial earlier this month, after a patient became sick. The trial has now restarted in the UK, but in the US, it's waiting on the go-ahead from the FDA.
"Stopping a trial in a vaccine programme is not uncommon and if you place safety at the centre of what you do, you're going to have to stop and look at events.
"Typically, the clinical guidelines recommend you don't disclose patient-level information or much information at all because you could compromise the study.
"We're looking at how much transparency we can provide... as an industry without compromising patient privacy or the trial itself."
"At the end of the day, people have to accept that they have to trust someone... So many regulators will look at this data and these results with different eyes... Medicine shouldn't be practiced by the media, it should be practiced by experts."
Trust is everything, said Gerberding. “We need to involve opinion leaders and trusted doctors at the local level to communicate the actual facts and stand strong.”
“It’s an understandable concern that in our race to get additional population protection, safety shortcuts will occur. That is not the case. The major vaccine manufacturers recently signed a pledge promising that they would adhere to safety requirements of the regulatory agents and not jump ahead of the curve in an effort to win the race.
“We are self-policing and making sure we don’t overstep that confidence barrier.”
Preparing for the next one
We can’t afford to be short-termist while we’re focusing on COVID-19, Gerberding and Berkley also warned.
“It is evolutionarily certain we will have more outbreaks,” said Berkley. “The way to build back better is to have routine systems and continued investment. The cost [of COVID-19] has been between $9 and $12 trillion. A little bit of investment in peacetime is exactly the right thing to do.
Gerberding added: "We have to make sure that while we're fighting this pandemic, we're preparing for the next, because I do believe it's only a matter of time before we face another of these situations or potentially something worse."
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Stay up to date:
Vaccination
Related topics:
Forum Stories newsletter
Bringing you weekly curated insights and analysis on the global issues that matter.
More on Health and Healthcare SystemsSee all
Mansoor Al Mansoori and Noura Al Ghaithi
November 14, 2025