Why pharma and life sciences must act now on the quantum threat

The stakes are high for pharma and life sciences. Image: Getty Images/iStockphoto
- Quantum computing technology threatens to upend the cryptographic foundations that underpin modern cybersecurity.
- Stakes are high for pharma and life sciences, due to their reliance on clinical trials, intellectual property and patient health data.
- Organizations in the sector must act now to protect future medical breakthroughs from being undone by future quantum risks.
History has shown that no form of encryption lasts forever. The simple substitution ciphers used by the Romans were eventually cracked by rivals who spotted their patterns. Fast forward to the 20th century and the Enigma machine, once thought to be unbreakable, was deciphered by the codebreakers at Bletchley Park with Alan Turing and his colleagues changing the course of the Second World War.
The story is always the same: new technology eventually outpaces old defences. As computers have grown more powerful and more affordable, their use has spread well beyond research labs and businesses to adversaries and criminals, who have been quick to weaponize that same progress.
Today, we stand at the brink of another paradigm shift: quantum computing, which threatens to upend the cryptographic foundations that underpin modern cybersecurity.
What is the quantum threat?
Quantum computing is a double-edged sword. In the right hands, it could accelerate breakthroughs such as drug discovery, but in the wrong hands, it could shatter digital trust.
Once cryptographically relevant quantum computers (CRQCs) arrive, widely used algorithms like RSA and elliptic curve cryptography (ECC) may collapse, potentially enabling attackers to forge digital signatures, break secure channels and decrypt sensitive data. These attacks are not hypothetical – algorithms like Shor’s and Grover’s have already demonstrated how quantum machines could break today’s encryption.
For pharmaceutical and life sciences (PLS) organizations, the stakes are especially high. Clinical trials, intellectual property and patient health information depend on encryption that quantum computing could render obsolete.
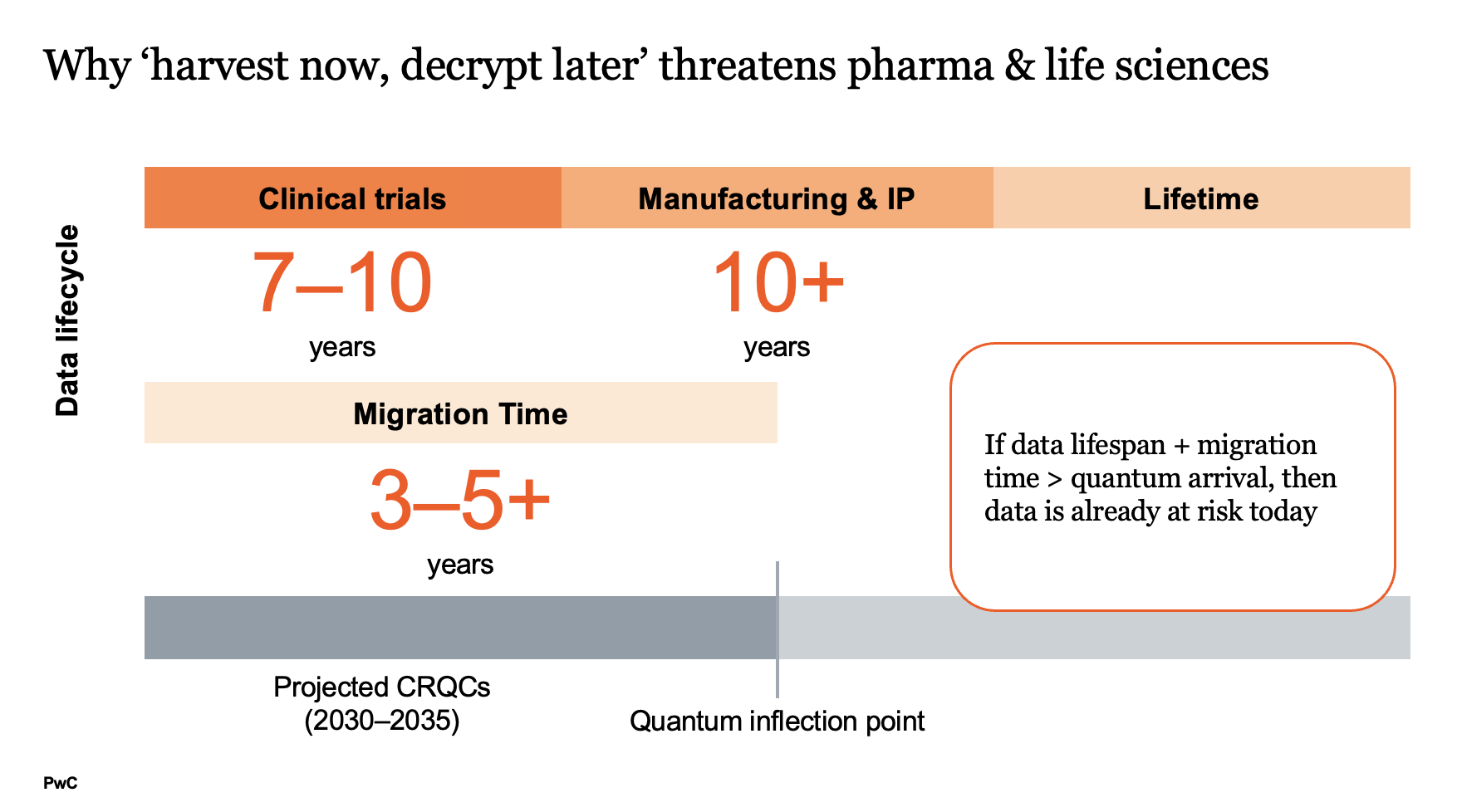
Adversaries including nation states are likely already pursuing “harvest now, decrypt later” strategies, collecting encrypted research, manufacturing data and genomic information today once CRQCs emerge.
With CRQCs expected by 2030 to 2035, and migrations to post quantum cryptography (PQC) requiring years of preparation, the time to act is now. For data that must remain secure for decades, this is not a future concern but an immediate vulnerability.
Why pharma and life sciences are at risk
All industries are exposed to quantum risk, but stakes are especially high for pharmaceutical and life sciences organizations.
Long data lifecycles, regulatory filings, patient safety and the potential loss of intellectual property and trade secrets creates not only scientific and operational challenges, but also severe reputational, financial and legal consequences. The sector’s reliance on global collaboration with contract research organizations, regulators and academic partners further broadens the attack surface.
A decade-long clinical trial overlaps with projected CRQC timelines, putting sensitive results within reach of adversaries. Proprietary vaccines, drug formulas and manufacturing processes and protocols represent billions in investment and are prime targets for reverse engineering. Genomic and patient data, once decrypted, could trigger regulatory violations and lasting reputational damage.
In a sector where patient safety and data integrity are paramount, quantum readiness could become a competitive differentiator. Furthermore, pharma’s role in national security and pandemic response makes it a high-value target for state-sponsored quantum espionage.
The following type of PLS information is most at risk:
- Clinical trial and R&D data: Long timelines leave data exposed
- Intellectual property: Molecules, formulations and trade secrets drive industry value
- Patient health information: Breaches carry legal, ethical and trust implications
- Third parties: Weak supplier security magnifies exposure
Why act now on the quantum threat
For the pharmaceutical and life sciences sector, information created today can remain valuable for the next decade, making it a prime target for long-term threats.
Transitioning to post quantum cryptography takes years and updating certificates, protocols and systems cannot wait until quantum computers arrive. Regulators are beginning to demand transition roadmaps, adding external pressure.
Meanwhile, the global pool of skilled cryptographers and quantum-aware cybersecurity professionals is limited. Organizations that delay taking actions will find themselves competing for scarce expertise.
The journey to quantum resilience is complicated by evolving standards, infrastructure bottlenecks and governance challenges. The US National Institute of Standards and Technology (NIST) is still finalizing post quantum cryptography algorithms, and organizations must plan to stay agile as the latest updates emerge.
In addition, PQC keys are larger and may strain legacy systems. Even without post quantum cryptography, millions of certificates will require renewal over the next decade, mostly due to the reduced 47-day renewal cycle expected by 2029, creating potential bottlenecks if processes are not automated. Shadow IT and unmanaged cryptography add further blind spots and risks.
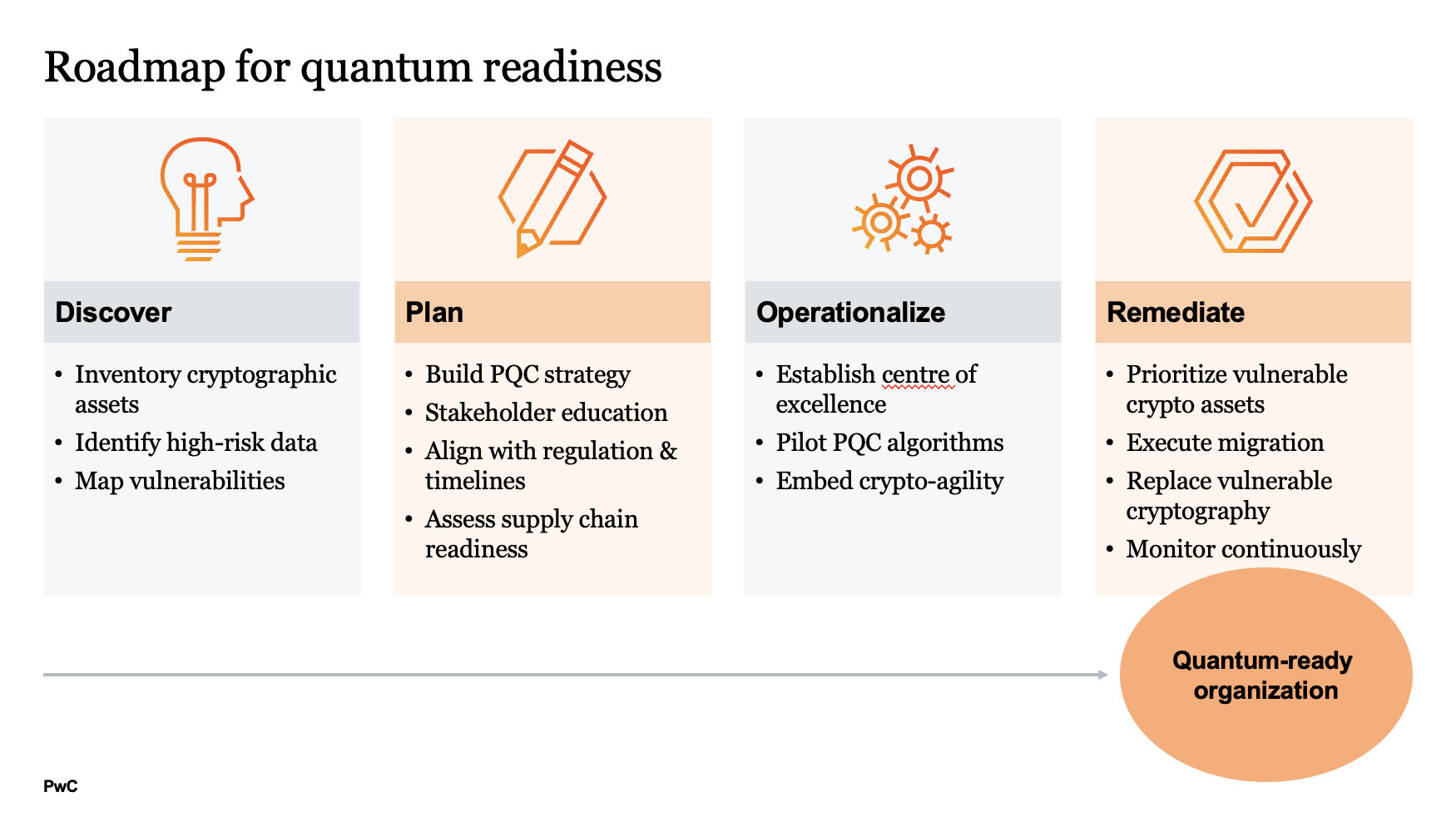
Roadmap for quantum readiness
Tech evolves at an incredible pace. For the benefit of patients (and shareholders), pharmaceutical and life science organizations must stay agile, curious, responsible and collaborative.
PLS organizations can reduce risk by taking a phased, structured approach. Migration requires balancing near-term protection with long-term transformation.
Quantum resilience as a strategic imperative
Quantum resilience is not merely a technical concern – it is a strategic and programmatic shift. It touches innovation, trust and competitiveness. Boards and leadership teams must make post quantum cryptography a top priority to safeguard the sector’s future.
A quantum-enabled breach could compromise not only intellectual property and trial results but also public trust in life sciences. Organizations that act today will secure not just their own assets, but also the integrity of the global healthcare system, and we all have a responsibility to play our part in protecting it.
In short, pharmaceutical and life sciences organizations sit at the intersection of innovation and trust. Preparing now helps protect tomorrow’s medical breakthroughs from being undone by future quantum risks.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Stay up to date:
Cybersecurity
Related topics:
Forum Stories newsletter
Bringing you weekly curated insights and analysis on the global issues that matter.
More on CybersecuritySee all
Anna Sarnek and Michael Brett
February 20, 2026