Large quantitative models: How AI is accelerating drug discovery
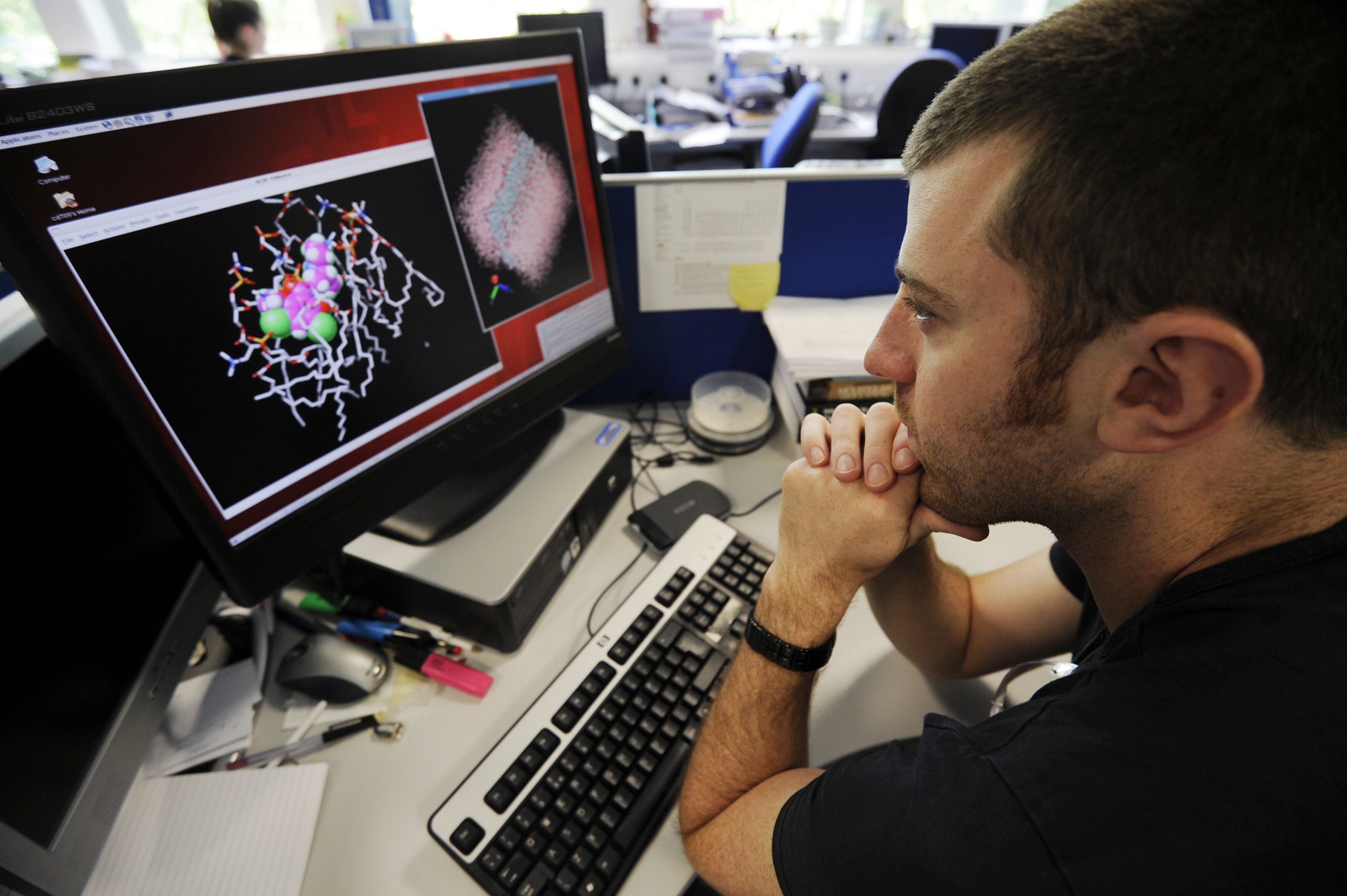
Using large quantitative models could save billions in R&D Image: REUTERS/Paul Hackett
- Large artificial intelligence driven quantitative models are accelerating drug discovery, with one research-tech collaboration compressing neurodegenerative disease research timelines from years to months.
- Using large quantitative models could save billions in research and development, reduce reliance on animal testing and expand access to treatments for rare and complex diseases.
- SandboxAQ and UCSF’s Institute for Neurodegenerative Diseases were recognized at AMNC 2025 as part of MINDS’ inaugural cohort, a World Economic Forum programme highlighting value-creating AI applications.
Even with today’s modern technologies and scientific approaches, advancing a new drug therapy from concept to clinic can average 10 years and cost over $2.5 billion, with a staggering 90% of candidates failing in pre-clinical and clinical phases.
These metrics are particularly unfavourable for diseases with poorly understood biology or where animal models inadequately predict human outcomes. The result is that patients endure long waits for innovations that frequently never reach them, while investment in rare or complex disorders has lagged due to the substantial risks and limited returns.
Medicines that are approved typically come with exorbitant price tags, as manufacturers seek to recover their losses from unsuccessful ventures and rebuild their research and development (R&D) coffers for future initiatives.
...major academic, commercial and non-profit biopharma research organizations around the world are already leveraging large quantitative models to advance research pipelines in oncology, biomarker discovery and additional difficult-to-treat disorders
”Clearly, the current system is broken and unsustainable. A better approach is needed to significantly reduce the time, cost and risk of developing new therapies, much of which can be attributed to trial-and-error wet-lab experimentation and the lack of accurate data or insights that drive discoveries.
Artificial intelligence (AI) has shown tremendous promise in accelerating certain aspects. However, training language-based AI models on existing literature, historical data, and failed experiments is unlikely to help us discover novel molecules and unknown compounds that will unlock breakthroughs in new treatments for the most challenging and complex diseases.
To achieve this, we need new AI models that can go beyond our existing pre-clinical data and literature, enabling deeper exploration of chemical space to uncover new candidates. They need to understand the nature and behaviour of molecules and proteins so they can simulate their interactions and predict potential outcomes without lengthy and costly physical experimentation.
They also need to generate new data from these simulations to fill in knowledge gaps, guide their experiments and help them make clinical decisions faster and more effectively.
Addressing entrenched challenges in drug discovery
Neurodegenerative diseases such as Parkinson’s and Alzheimer’s have long resisted therapeutic breakthroughs. Drug development in these areas remains one of the most difficult and costly undertakings in modern medicine:
- Tens of billions of dollars are spent on drug development.Timelines often span over a decade.
- There have been repeated failures to discover drugs that slow or stop the progression of disease.
- The traditional model is no longer sustainable, particularly in light of the urgent and growing needs of patients.
A recent collaboration between the Institute for Neurodegenerative Diseases at the University of California San Francisco (UCSF) and advanced computing firm SandboxAQ signals a paradigm shift.
Leveraging a new class of AI known as large quantitative models, UCSF has not only accelerated its research efforts but also redefined what is possible in the development of novel therapeutics.
This case study highlights how the AQBioSim platform is fundamentally transforming the drug discovery process, delivering unprecedented speed, efficiency and success.
For years, the development of neurodegenerative therapies has been hindered by the limited availability of computational tools and inefficient screening techniques. Progress was slow and expensive, with researchers relying on brute-force screening of hundreds of thousands of compounds, typically yielding a handful of weak leads after months of work.
Despite advances in automation and bioinformatics, the core bottlenecks in modelling and prediction remained.
Under the leadership of Nobel Laureate Dr Stanley Prusiner, a team at UCSF confronted these same challenges. In 2024, the team projected that a promising Parkinson’s therapy would not be ready for clinical trials until 2031. After exploring traditional computational platforms without success, Dr Prusiner turned to SandboxAQ.
He was searching for an AI solution that could do more than augment productivity; he needed a platform that could materially de-risk and accelerate drug discovery and development.
Expanding therapeutic reach and impact
The implications of these advances reach far beyond neurodegenerative diseases. Large quantitative models are adaptable to oncology, genetics and a wide range of rare diseases, providing the foundation for rapid drug development pipelines even with limited experimental data or complex biological targets.
It could also help biopharma organizations comply with the US Food and Drug Administration’s recently released guidance phasing out animal testing requirements for monoclonal antibodies and other drugs.
Scaling this innovation across biopharma could significantly shift the industry’s productivity curve. In 2025, over 6,000 companies and research groups globally are engaged in drug discovery, according to Citeline.
Accelerating R&D for new Alzheimer’s could impact tens of millions of people impacted by the disease – expected to reach 78 million people by 2030.
Similarly, a 2021 Global Burden of Disease Study found that 11.8 million people worldwide had Parkinson’s disease. A March 2025 study by The BMJ Group estimates Parkinson’s will grow to more than 25 million by 2050 – a 112% increase from 2021.
How the Forum helps leaders strengthen health systems through collaboration
Just as importantly, advances in in-silico screening and optimization (performed using computer modelling and simulation) could save billions of dollars in inefficient, risk-based R&D spending annually, leading to greater investment in therapies for challenging or rare diseases, including those affecting small or underserved populations.
At UCSF, our researchers can now profile millions of molecules monthly, empowering integrated teams working on diseases ranging from Alzheimer’s and Parkinson’s disease to newly characterized misfolded proteins.
Other major academic, commercial and non-profit biopharma research organizations around the world are already leveraging large quantitative models to advance research pipelines in oncology, biomarker discovery and additional difficult-to-treat disorders.
Looking ahead to a healthier future
The UCSF-SandboxAQ collaboration is a proof point for how multi-disciplinary, physics-native, quantitative AI tools can disrupt and transform decades-old drug discovery practices, leading to new innovations and clinical breakthroughs at scale.
The approach offers researchers and industry leaders a blueprint for overcoming longstanding obstacles – notably high cost and risk, data sparsity and high rate of clinical failure – paving the way for transformative therapies and relief to millions of patients worldwide.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Stay up to date:
Artificial Intelligence
Related topics:
Forum Stories newsletter
Bringing you weekly curated insights and analysis on the global issues that matter.
More on Health and Healthcare SystemsSee all
Elizabeth Mills and Gayle Markovitz
January 30, 2026