COVID-19: What you need to know about the coronavirus pandemic on 23 December

Researchers find the Omicron COVID-19 variant is less likely to lead to patients needing hospital treatment. Image: REUTERS/Yves Herman
Explore and monitor how COVID-19 is affecting economies, industries and global issues

Get involved with our crowdsourced digital platform to deliver impact at scale
Stay up to date:
COVID-19
- This daily news round-up brings you a selection of the latest news and updates on the COVID-19 coronavirus pandemic, as well as tips and tools to help you stay informed and protected.
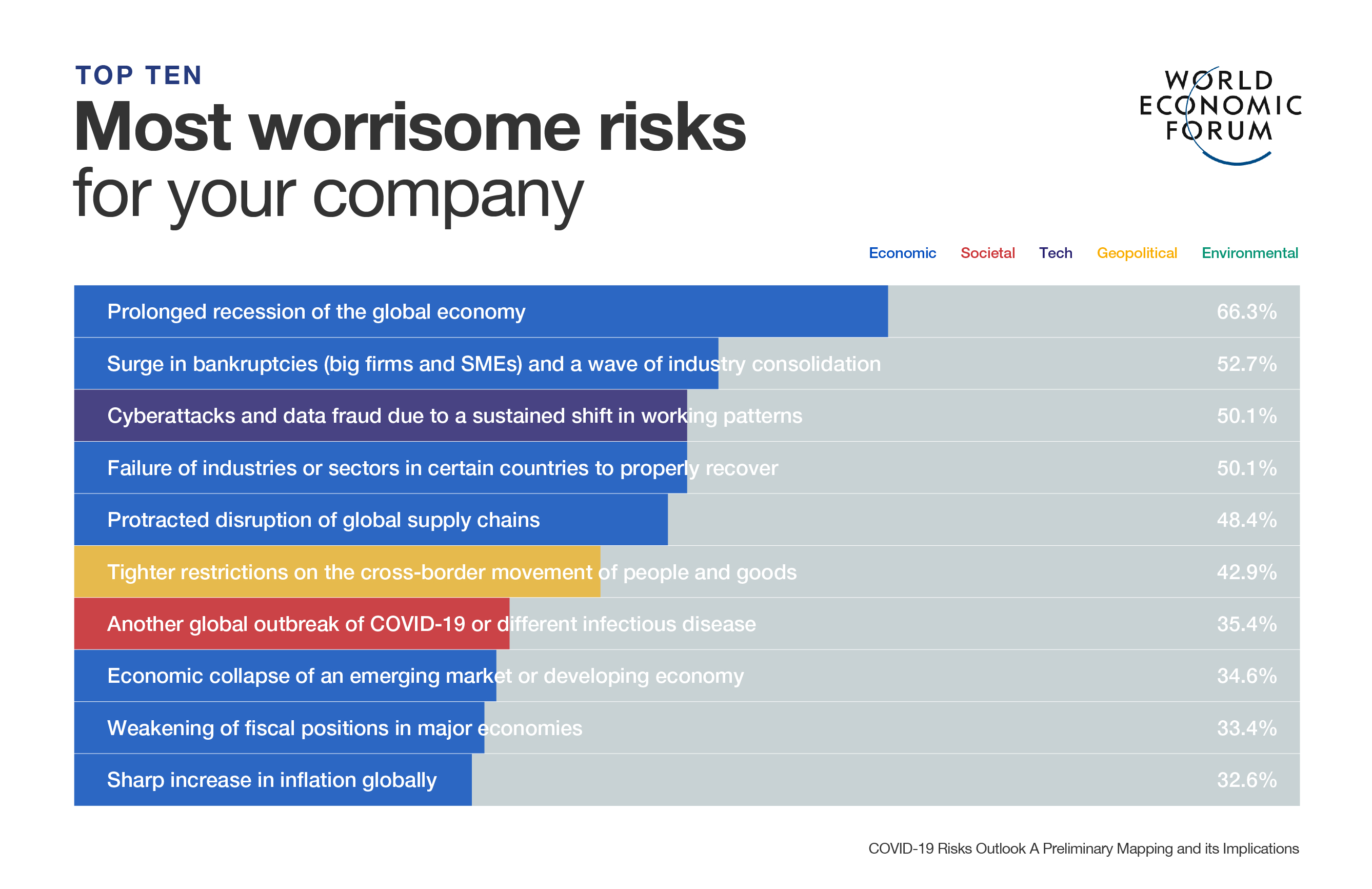
- Top stories: Studies suggest Omicron variant leads to milder illness; Australia brings back restrictions as new infections surge; US approves Pfizer COVID-19 pill for home use.
1. How COVID-19 is affecting the globe
Confirmed cases of COVID-19 have passed 277.1 million globally, according to Johns Hopkins University. The number of confirmed deaths has now passed 5.37 million. More than 8.85 billion vaccination doses have been administered globally, according to Our World in Data.
Australia reintroduced COVID-19 curbs such as mandated mask wearing indoors, capacity limits and QR code check-ins to cover most of the population on Thursday as daily infections hit a fresh record, fuelled by the highly infectious Omicron variant. The changes for 17 million people two days before Christmas mark a reversal of the country's plans for a permanent reopening.
Britain reported more than 100,000 new daily COVID-19 cases for the first time since widespread testing was introduced, with 106,122 on Wednesday compared with 90,629 on Tuesday. The rapid spread of the Omicron variant has driven a surge in cases, according to government data. Britain also said it would start vaccinating vulnerable children aged five to 11 against COVID-19.
The Chinese city of Xian has imposed a new lockdown and issued travel restrictions on its 13 million residents. A new COVID-19 outbreak has seen community cases tick higher. The daily count of domestically transmitted COVID-19 infections with confirmed symptoms in Xian has increased for six consecutive days since December 17.
India's capital New Delhi has banned Christmas parties and other celebrations ahead of New Year to contain a possible surge in the Omicron coronavirus variant, as the city reported the country's highest number of cases along with Maharashtra state. India has reported no deaths from the variant so far. Its health minister has said most known cases are asymptomatic.
France has cancelled its order for Merck & Co's COVID-19 antiviral drug following disappointing trial data. It hopes instead to receive Pfizer's competing drug before the end of January, the health minister said on Wednesday.
Nigeria destroyed more than a million doses of expired AstraZeneca vaccines on Wednesday in a bid to assure a wary public that they have been taken out of circulation. The destruction came more than a week after health authorities said some COVID-19 doses donated by Western nations had a shelf life that left only weeks to administer the shots.

2. Two studies suggest lower risk of hospitalization with Omicron versus Delta
The risk of needing to stay in hospital for patients with the Omicron variant of COVID-19 is 40% to 45% lower than for patients with the Delta variant, according to research by London's Imperial College.
"Overall, we find evidence of a reduction in the risk of hospitalization for Omicron relative to Delta infections, averaging over all cases in the study period," the researchers said of the study, which analyzed data from PCR-test confirmed cases in England between December 1 and December 14.
Scientists are racing to answer questions about the virulence and severity of Omicron to help governments respond to the variant, which is spreading at breakneck speed.
The British research follows a South African study on Wednesday which found that people diagnosed with Omicron in South Africa between October 1 and November 30 were 80% less likely to be admitted to hospital than those diagnosed with another variant in the same period.
Imperial College researchers said the risk of any visit to hospital with Omicron was between 20% and 25% lower than with Delta.
What is the World Economic Forum doing to manage emerging risks from COVID-19?
3. US authorizes Pfizer oral COVID-19 treatment, first for at-home use
The United States has authorized Pfizer Inc's antiviral COVID-19 pill for people aged 12 and older at risk of severe illness, the first oral and at-home treatment as well as a new tool against the fast-spreading Omicron variant.
Pfizer's antiviral regimen, Paxlovid, was nearly 90% effective in preventing hospitalizations and deaths in patients at high risk of severe illness, according to data from the company's clinical trial. Recent lab data suggests the drug retains its effectiveness against Omicron, Pfizer said.
Pfizer raised its 2022 production projections to 120 million courses of treatment from 80 million and said it was ready to start immediate delivery in the United States. The treatment's two-drug regimen includes a new medicine and a second older antiviral called ritonavir.
The US government will have 265,000 treatment courses available by January and supply will ramp up in subsequent months, White House COVID-19 response coordinator Jeff Zients told a briefing. The government expects to receive the 10 million courses it has ordered within six months.
"Paxlovid's approval is a major milestone that marks another step towards making COVID-19 a much more manageable infection," said Amesh Adalja, a senior scholar at the Johns Hopkins Institute for Health Security.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Related topics:
The Agenda Weekly
A weekly update of the most important issues driving the global agenda
You can unsubscribe at any time using the link in our emails. For more details, review our privacy policy.
More on COVID-19See all
Charlotte Edmond
January 8, 2024
Charlotte Edmond
October 11, 2023
Douglas Broom
August 8, 2023
Simon Nicholas Williams
May 9, 2023
Philip Clarke, Jack Pollard and Mara Violato
April 17, 2023